Answer:
1g Hydrogen
Step-by-step explanation:
Getting to the equation:
Calcium in water reacts vigorously to give a cloudy white Precipitate (compound) called Calcium hydroxide alongwith the evolution of Hydrogen gas.

- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Balancing the equation:
This reaction is not in it's balanced form! The number of atoms of Hydrogen on the left is 2 while that on the right is 4,I.e.,they're not equal.
Adding a 2 in front of H2O solves the problem by making the number of atoms of each element on both the sides equal.
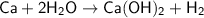
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Observations:
Looking into the equation more carefully, we see:
1 atom of Calcium reacts with 2 molecules of water to give 1 molecule of Calcium Hydroxide alongwith 1 molecule of Hydrogen gas.
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Gram atomic and molecular masses
Mass of one atom of Calcium = it's gram atomic mass
= 40 g
Mass of one "molecule" of Hydrogen
= it's Gram molecular mass
= gram mass of one atom × number of atoms in one molecule
= 1 × 2
= 2 g
So,
according to our observation:
One atoms of Calcium gives one molecule of Hydrogen (during the particular reaction)
=> 40g of Calcium gives = 2g of Hydrogen
•°• 1 g of Calcium gives =
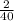
=
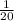 g Hydrogen
g Hydrogen
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Answer:
We're provided with 20g of Calcium,
=> 20g of Calcium gives = 20 ×
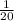 g H2
g H2
= 1 g H2
_______________
Hope this helps!