Answer:
About 547 grams.
Step-by-step explanation:
We want to determine the mass of copper (II) bicarbonate produced when a reaction produces 2.95 moles of copper (II) bicarbonate.
To do so, we can use the initial value and convert it to grams using the molar mass.
Find the molar mass of copper (II) bicarbonate by summing the molar mass of each individual atom:
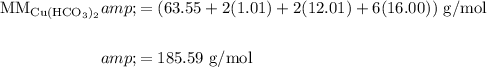
Dimensional Analysis:
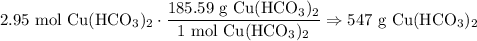
In conclusion, about 547 grams of copper (II) bicarbonate is produced.