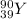Answer:
Y-90
Step-by-step explanation:
In a
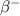 decay, the nucleus emits an electron and an electron antineutrino, thus causing the atomic number of the nucleus to increase by one while its mass number remains the same. Therefore, the new nucleus after the decay is
decay, the nucleus emits an electron and an electron antineutrino, thus causing the atomic number of the nucleus to increase by one while its mass number remains the same. Therefore, the new nucleus after the decay is