Answer:

Step-by-step explanation:
We are asked to find how much heat a sample of copper absorbs when the temperature is increased.
Since we know the mass, temperature increase, and specific heat capacity, we can use the following formula to calculate heat.
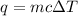
The mass of the copper sample is 100 grams, the temperature is changed or increased by 30.0 degrees Celsius, and the specific heat of copper is 0.39 Joules per gram degrees Celsius.
- m= 100 g
- c= 0.39 J/g °C
- ΔT= 30.0 °C
Substitute the values into the formula.
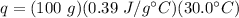
Multiply the first two values. Note that the units of grams cancel.
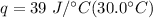
Multiply again, this time the units of degrees Celsius cancel.
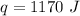
The copper sample absorbs 1170 Joules of heat and Choice B is correct.