Solution :
1 lb = 453.592 g
1 gallon = 3785 g
For every 5 gallon gasoline = 5 lb of C is found
= 5 x 453.592 g of C atoms
= 2267.96 g of C atoms
Assume the consumption of car = 15 miles per kg of gasoline
The amount of gasoline used per year
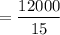
= 800 kg
In gallons =
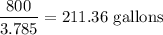
5 gallons will produce = 2267.96 g of C atoms
Therefore,
211.36 gallons will produce =
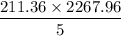
= 95871.21 g
= 95.87 kg
or = 25.32 gallon