Answer:
d the oxygen escapes.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out firstly convenient for us to write out the chemical reaction taking place:
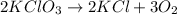
Thus, since both potassium chlorate and chloride are solid whereas oxygen is gaseous, it tends to scape as we are working in an open test tube and that is why the products side do not have the reactants, KCl and O2, to return the reactants side, KClO3 in agreement to the concept of equilibrium reaction. This situation can be solved by working in a closed container.
Therefore the answer is d the oxygen escapes.
Regards!