Answer:
(a) m = 0.327 m.
(b) m = 4.57 m.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to solve this problem by firstly considering the fact that the molality is computed by dividing the moles of solute by the kilograms of solvent, in this case water; in such a way, we proceed as follows:
(a) We firstly calculate the moles of 36.2 grams of sucrose as its molar mass is 342.3 g/mol:
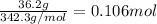
Next, the kilograms of water in this case are 0.323 kg so that the molality will be:
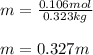
(b) In this case, we directly realize that the kilograms of water are now 1.889 kg so that the molality will be:
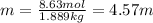
Clearly, the both of them in molal, m, units.
Regards!