Answer:
Manganese decreases from 4+ to 2+ (reduced and oxidizing agent) and nitrogen increases from 2+ to 5+ (oxidized and reducing agent).
Step-by-step explanation:
Hello there!
In this case, according to the given redox reaction, we rewrite it as a convenient first step:
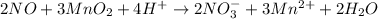
Next, we assign the oxidation numbers as follows:
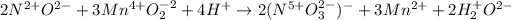
Thus, we can see that both manganese and nitrogen undergo a change in their oxidation number, the former decreases from 4+ to 2+ (reduced and oxidizing agent) and the latter increases from 2+ to 5+ (oxidized and reducing agent).
Regards!