Answer: The reaction,
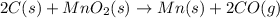 is a single-displacement reaction.
is a single-displacement reaction.
Step-by-step explanation:
A chemical reaction in which one element of a compound is replaced by another element participating in the reaction.
For example,
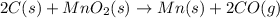
Here, the element manganese is replaced by carbon atom. As only one element gets replaced so, it is a single-displacement reaction.
Thus, we can conclude that
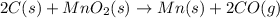 is a single-displacement reaction.
is a single-displacement reaction.