Answer:
Indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium after reactant or product is added.
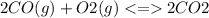
Step-by-step explanation:
When the reactants concentration increases, then the equilibrium will shift towards products and when the concentration of products increases, then equilibrium will shift towards reactants.
So, increases in concentration of carbon monoxide (CO) shifts the equilibrium to favor the formation of carbondioxide.
Similarly increase in concentration of oxygen also favor the formation of product carbon dioxide.
Increase in concentration of CO2 favors the formation of CO and O2.
Decrease in product concentration also favors the formation of product.
Decrease in reactant concentration favors the formation of reactants only.