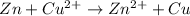Answer: The correct option is D.
Step-by-step explanation:
Redox reaction is defined as the reaction in which oxidation and reduction take place simultaneously. It is known as the reaction in which the exchange of electrons takes place.
The oxidation reaction is defined as the reaction in which a chemical species loses electrons in a chemical reaction. It occurs when the oxidation number of a species increases.
A reduction reaction is defined as the reaction in which a chemical species gains electrons in a chemical reaction. It occurs when the oxidation number of a species decreases.
From the given ionic reactions:
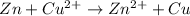
On the reactant side:
Oxidation number of Zn = 0
Oxidation number of Cu = +2
On the product side:
Oxidation number of Cu = 0
Oxidation number of Zn = +2
As the oxidation number of Zn is increasing from 0 to +2. Thus, it is getting oxidized. Similarly, the oxidation number of Cu is decreasing from +2 to 0. Thus, it is getting reduced. Therefore, forming a redox couple
Hence, the correct option is D.