Answer:
M = 3.69 M.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the molar concentration of the 1.29 moles of KCl in 350 mL of solution by recalling the mathematical definition of molarity as the division of the moles by the volume in liters, in this case 0.350 L; thus, we proceed as follows:
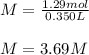
Which gives molar units, M, or just mol/L.
Regards!