Answer:
NH3 is the base and NH4Cl is the salt.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it is possible for us to rewrite the chemical equation and thus obtain:
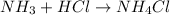
Whereas it is possible to notice that ammonia, NH3, received the hydrogen ions from HCl to form NH4 ions and Cl ions; in such a way, we infer that NH3 is the base and NH4Cl is the salt.
Regards!