Answer: If you want to make 10 moles of
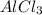 then 30 moles of HCl are required in the given reaction.
then 30 moles of HCl are required in the given reaction.
Step-by-step explanation:
The given reaction equation is as follows.
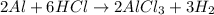
Here, 6 moles of HCl reacts with 2 moles of
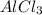 . This means that 1 mole of
. This means that 1 mole of
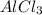 is obtained as follows.
is obtained as follows.
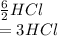
Hence, 3 moles of HCl will give 1 mole of
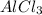 . So, moles of HCl required to react with 10 moles of
. So, moles of HCl required to react with 10 moles of
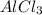 is as follows.
is as follows.
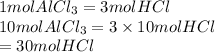
Thus, we can conclude that if you want to make 10 moles of
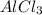 then 30 moles of HCl are required in the given reaction.
then 30 moles of HCl are required in the given reaction.