Answer:

Step-by-step explanation:
In this problem, the temperature stays constant. The volume and pressure change, so we use Boyle's Law. This states that the pressure of a gas is inversely proportional to the volume. The formula is:
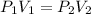
Now we can substitute any known values into the formula.
Originally, the gas has a volume of 25.0 liters and a pressure of 2.05 atmospheres.
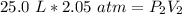
The volume is decreased to 14.5 liters, but the pressure is unknown.
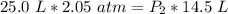
Since we are solving for the new pressure, or P₂, we must isolate the variable. It is being multiplied by 14.5 liters and the inverse of multiplication is division. Divide both sides by 14.5 L .
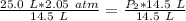
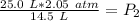
The units of liters cancel.
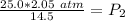
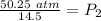
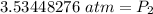
The original values of volume and pressure have 3 significant figures, so our answer must have the same.
For the number we found, that is the hundredth place.
The 4 in the thousandth place (in bold above) tells us to leave the 3 in the hundredth place.
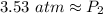
The new pressure is approximately 3.53 atmospheres.