Answer:
The correct answer is option A: they are isotopes.
Step-by-step explanation:
From atom X we know that the number of protons is 7 and the number of neutrons is 7 and from atom Z we know that the number of protons is 7 and the number of neutrons is 8.
Since the number of protons of atom X and atom Z is the same, we have that atom X and atom Z is the same element. The difference in the number of neutrons tells us that atom X and atom Z are isotopes. Remember that an isotope is one element that has atoms with different numbers of neutrons.
The mass number is given by:
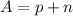
Where n is the number of neutrons and p is the number of protons.
For atom X and atom Z we have:
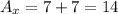
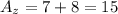
Hence, they have a different mass number.
We know that the element with 7 protons is nitrogen. The first isotope is
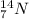 and the second isotope is
and the second isotope is
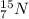 . Both isotopes are stables (they are not radioactive).
. Both isotopes are stables (they are not radioactive).
Therefore, the correct answer is option A: they are isotopes.
I hope it helps you!