Answer:
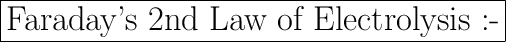
According to the second law of electrolysis, the same quantity of electricity will produce or dissolve chemically equivalent amounts of all the substances. This quantity of electricity is called Faraday (F). One Faraday is equal to 96487 coulombs per mole of electronic charges.