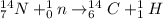Answer: The complete reaction is as follows.
Step-by-step explanation:
When nucleus of two or more atoms are bombarded together then it leads to the formation of new particles with new identity. This type of reaction are called nuclear reaction.
For example,
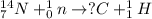
Here, nitrogen atom when bombarded with a neutron then it is forming hydrogen and a carbon atom.
As total atomic mass on reactant side is (14 + 1) = 15
So, the atomic mass of carbon formed on product side is (15 - 1) = 14.
The number of protons holded by this carbon atom is (7 - 1) = 6.
Therefore, we can conclude that the complete reaction is as follows.