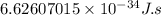Answer:
"h" signifies Planck's constant
Step-by-step explanation:
In the equation energy E = h X v
The "h" there signifies Planck's constant
Planck's constant is a value, that shows the rate at which the energy of a photon increases/decreases, as the frequency of its electromagnetic wave changes.
It was named after Max Planck who discovered this unique relationship between the energy of a light wave and its frequency.
Planck's constant, "h" is usually expressed in Joules second
Planck's constant =