Answer:
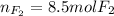
Step-by-step explanation:
Hello there!
In this case, since the reaction is not given and may find thousands of options for obtaining potassium chloride, we may assume that the undergoing reaction is the formation of potassium fluoride from elemental potassium and fluorine:
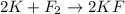
Whereas we can find a 1:2 mole ratio of fluorine to potassium fluoride; in such a way, given 17 moles of the latter, we can find the moles of the former as shown below:
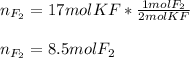
Regards!