Solution :
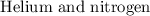 gases are contained in a conduit
gases are contained in a conduit
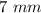 is diameter and
is diameter and
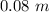 long at 317 K (44°C) and a uniform constant pressure of 1 atm.
long at 317 K (44°C) and a uniform constant pressure of 1 atm.
Given :
Diameter, D = 7 mm
L = 0.1 m
T = 317 K
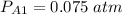
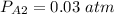
P = 1 atm
From, table
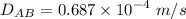
We know :
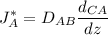
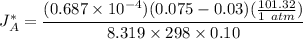
=
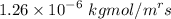
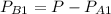
= 1 - 0.075
= 0.925 atm
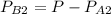
= 1 - 0.03
= 0.97 atm
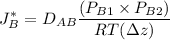
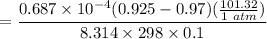
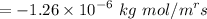
Partial pressure of helium
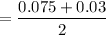
= 0.0525 atm