Answer:
e. None of these.
Step-by-step explanation:
Hey there!
In this case, since the Henry's law is defined in terms of pressure, henry's constant and pressure, as shown below:
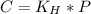
Whereas C is the concentration, KH the Henry's constant and P the pressure, we infer that the concentration of a gas solution is directly proportional to the pressure, which is not the group choices, therefore, the answer is e. None of these.
Best regards!