Answer: If a reaction releases 675,000 Joules then it means it releases 161.328872 kcal.
Step-by-step explanation:
According to the standard conversion,
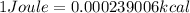
Therefore, 675,000 Joules will be converted into kcal as follows.
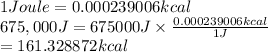
Thus, we can conclude that if a reaction releases 675,000 Joules then it means it releases 161.328872 kcal.