Answer:
M = 12.0M
Step-by-step explanation:
Hello there!
In this case, according to the given by-mass percent of HCl, which can be set up in terms of mass of HCl over mass of solution, we can calculate the molarity, by multiplying by the density to get the mL's of solution and further convert to liters. Moreover, the molar mass of HCl (36.46 g/mol) must be also used to calculate the moles, since molar units requires moles of solute and liters of solution as shown below:
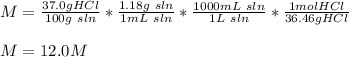
Best regards!