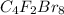Answer: The formula for tetracarbon difluorine octabromide is
Step-by-step explanation:
The naming of covalent compound is given by:
The less electronegative element is written first.
The more electronegative element is written second. Then a suffix is added with it. The suffix added is '-ide'.
If atoms of an element is greater than 1, then prefixes are added which are 'mono' for 1 atom, 'di' for 2 atoms, 'tri' for 3 atoms and so on.
Hence, the correct formula for tetracarbon difluorine octabromide is