Answer:
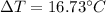
Step-by-step explanation:
Hello there!
In this case, given the mass and energy absorbed by water, it is possible to use the following equation in order to calculate the temperature change according to waters specific heat too (4.184 J/g°C):
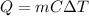
Therefore, solving for the temperature change we have:
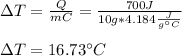
Which means that the temperature of water increases by 16.73 °C.
Best regards!