Yes, the eqⁿ is balanced.
Step-by-step explanation:
The reaction between hydrogen and oxygen results to the formation of water.
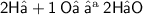
To check whether the eqⁿ is balanced, let us count the numbers in the reactant and product sides
L.H.S.
H = 2×2 = 4.
O = 2×1 = 2.
R.H.S.
H = 2×2 = 4
O = 2×1 = 2.
Hence it is a balanced chemical equation.