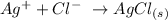Answer:
Step-by-step explanation:
Here, we want to get the net ionic equation of the given reaction
In reality, the given reaction produces silver chloride precipitate
Thus, the net reaction equation we would be writing is one that would give the silver chloride
We have this as: