Ok, so
First of all, let's remember what a mole is:
A mole can be described as the molecular mass of a compound or the mass of an element.
For example:
The molar mass of Zn is 65.38g. So, 1 mole of Zn equals to 65.38g of Zn. We can find the value of molar mass in the periodic table.
Now, we want to find the number of moles that are contained within 18.6g Zn.
For this, we could use the fact that:
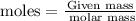
In this case, our given mass is 18.6g of Zn, and, the molar mass of Zn is 65.38g/mole. Then, if we replace:
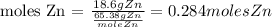
Therefore, there are 0.284 moles of Zn in 18.6g of Zn.