Answer:
297.85 K.
Explanation:
What is given?
Pressure 1 (P1) = 780 atm.
Pressure 2 (P2) = 851 atm.
Temperature 1 (T1) = 273 K.
Constant volume = 2.5 L.
What do we need? Temperature 2 (T2).
Step-by-step solution:
To solve this type of problem, when the volume is constant, but pressure and volume vary, we use Gay-Lusscac's Law. Gay-Lussac's law states that the pressure of a constant volume of a gas is directly proportional to its temperature. The formula of this law will look like this:
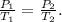
And finally, we have to solve for Temperature 2, which is T2, and replace the given data:
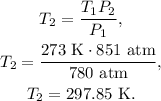
The final temperature would be 297.85 K.