1) Isotopes. Two atoms that have the same number of protons but a different number of neutrons.
2) Isotopes of Silicon.
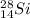
Mass number: 28
Atomic number: 14
Electrons: 14
Neutrons = mass number - atomic number = 14
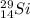
Mass number: 29
Atomic number: 14
Electrons: 14
Neutrons = mass number - atomic number = 15
3) Similarities and differences
These two isotopes of Silicon have the same number of protons and electrons.
14 protons and 14 electrons
These two isotopes of Silicon have different mass numbers and neutrons.
The first one, mass number 28 and 14 neutrons.
The second one, mass number 29 and 15 neutrons.
.