Answer:
The molar coefficient for Cu2S is 1.
Step-by-step explanation:
To balance the equation, it is important to make sure that there is the same amount of each element in the reactants side (to the left of the arrow) and in the products side (to the right of the arrow):
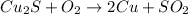
Now that the equation is balanced, the molar coefficient for Cu2S is 1.