The molarity can be calculated by the equation:
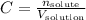
Since we know that we have 8.13 grams of NaCl, we can convert this mass to number of moles using the molar mass of NaCl:
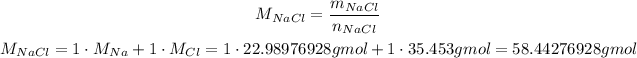
So, solving for the number of moles, we have:
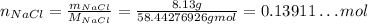
We also know the volume of the solution: 6.61 L, so:
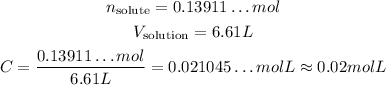
So, the molarity is approximately 0.02 mol/L.