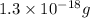The mass of a certain quantity of atoms of gold is directly proportional to the number of atoms of gold.
Thus, we can use rule of three to calculate the answer.
1 atom of gold has a mass of approximately 3.25 x 10⁻²² g.
4000 atoms of gold will have a mass of x.
1 atom ---- 3.25 x 10⁻²² g
4000 atoms ---- x
Thus:
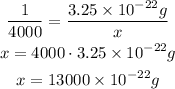
Now, we have to adjust the coefficient so that it comes back to scientific notation:
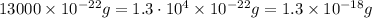
So, the mass is