We have that the Prevacid has 4.25*10^21 molecules.
Remember the Avogadro's Law, which says that 1 mol of a substance equals 6.022*10^23 molecules.
Based on this number, we can do the conversion like this:
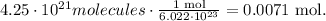
Using this result and the molar mass, you can find the mass:
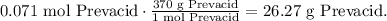
Then, the mass of 4.25*10^21 molecules of Prevacid is 26.27 g.*10