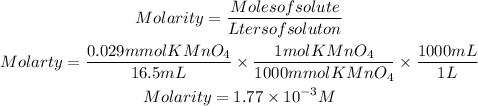We have an acid neutralization reaction that has the following balanced equation:
2KMnO4 + 5Na2C2O4 + 8H2SO4 => 8H2O + 10CO2 + K2SO4 + 2MnSO4 + 5Na2SO4
In this reaction we will focus on the moles of KMnO4 and Na2C2O4 (Sodium oxalate). The KMnO4 to Na2C2O4 ratio is 2/5.
We will do the following steps:
1. We find the moles of sodium oxalate using the mass found and the molar mass.
2. We find the moles of KMnO4 by stoichiometry.
3. We find the molarity of the solution by dividing the moles of KMnO4 by the volume of solution used.
4. The average molarity must be found by adding all the molarities and dividing by 3.
I will do the procedure for the first trial so you can replicate it with the rest.
Moles of sodium oxalate:
![\begin{gathered} MolNa_2C_2O_4=givengNa_2C_2O_4* MolarMassNa_2C_2O_4 \\ MolNa_2C_2O_4=0.098gNa_2C_2O_4/134gNa_2C_2O_4/1molNa_2C_2O_4=13.1 \\ MolNa_2C_2O_4=0.098gNa_2C_2O_4*(1molgNa_2C_4O_4)/(134gNa_2C_4O_4)*(1000mmolNa_2C_4O_4)/(1molNa_2C_2O_4)=0.073mmolNa_2C_2O_4 \end{gathered}]()
Moles of KMnO4 by stoichiometry:
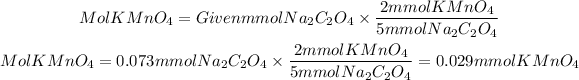
Molarity of the solution