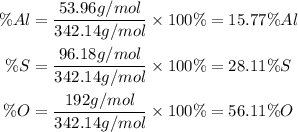To calculate the percent composition we must calculate the molecular mass of the molecule. To find each percent composition we divide the mass of the element in the molecule by the total mass of the molecule. Therefore we will have:
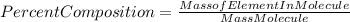
The molar mass of Al2(SO4)3 will be:
Al: 2 x 26.98 = 53.96 g/mol
S: 3 x 32.06 = 96.18 g/mol
O: 12 x 16 = 192 g/mol
Molar Mass= 53.96 + 96.18 + 192 =342.14g/mol
So, the percent composition will be: