STP stands for Standard Temperature and Pressure, so a gas in STP conditions are at a set of Temperature and Pressure that are standardized.
The STP are standards, so they can change. The STP changed years ago, but sometimes the old STP are used.
The old STP conditions were defined as 1 atm pressure and 273.15 K temperature. In such conditions, 1 mol of gas (assuming ideal gas) oocupies approximately 22.4 L.
The currect STP conditions are 1 bas pressure and 273.15 K temperature. In such conditions, 1 mol of gas (assuming ideal gas) occupies approximately 22.7 L.
Since we have options, we can test which STP the question is using.
We can do this as a rule of three.
Using the old STP, we have:
Volume ---- Number of moles
V ---- 0.7 mol
22.4 L ---- 1.0 mol
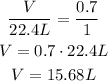
Using the new STP, we would have:
Volume ---- Number of moles
V ---- 0.7 mol
22.7 L ---- 1.0 mol
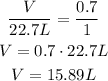
As we can see, although the correct STP to use currenctly is the second one, we only have answer for the old STP.
So, the correct option in this question is 15.68 L (using the old STP).