INFORMATION:
We know that:
- an element with atomic mass 201 undergoes alpha decay
And we must find the mass number in the resulting element
STEP BY STEP EXPLANATION:
To fin the mass number in the resulting element, we must know that
In general, during alpha decay, the atomic number (Z) is reduced by two, and the mass number (A), by four.
Now, knowing that the mass number is reduced by 4 and the atomic mass for our element is 201. Then, the atomic mass for the new element would be
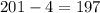
ANSWER:
The mass number in the resulting element is 197