Answer:
There are 0.186g of Xe.
Step-by-step explanation:
The information given in the exercise is:
- Volume: 35.0 mL (0.035L)
- Temperature: 298 K
- Pressure: 0.980 atm
1st) We can calculate the moles of the gas with the Ideal Gases formula, by replacing the values of volume, temperature and pressure.
Remember that the variables units must be atm, L, mol and Kelvin when using the Ideal Gases formula:
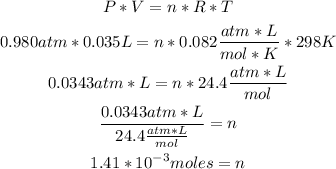
Now we know that there are 1.41x10^-3 moles of gas.
2nd) Finally, we have to convert the moles to grams, using the molar mass of Xe (132g/mol):
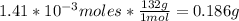
So, there are 0.186g of Xe.