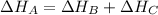Answer:
D) The enthalpy change for a reaction equals the sum of the enthalpy changes of each of its steps.
Step-by-step explanation:
Hess's Law explains that the enthalpy of a chemical reaction can be expressed as the sum of the enthalpies of the partial reactions that can occur to reach the main reaction.
For example, the sum of the previuos exercise: