ANSWER
The mass of titanium is 22.6 grams
OPTION B
Explanation
Given data:
The mole of titanium = 0.473 mole
Let x be the mass of titanium in grams
The mass of titanium can be calculated using the below formula
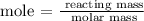
From the above formula, you can see that the molar mass of titanium is not given, and that can easily be determined using our periodic table.
From the periodic table, the molar mass of titanium is 47.867 g/mol.
The next thing is t substitute the value into the above formula
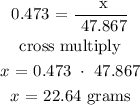
since x represents the mass of titanium in grams, therefore, the mass of titanium is 22.6 grams