Answer:
![2Mg+SO_2\operatorname{\rightarrow}2MgO+S]()
Explanations:
For a chemical equation to be balanced, the total moles at the reactant must be equal to that of the product. According to the given question, the reactants are magnesium and sulphur dioxide.
• Magnesium = Mg
,
• sulphur dioxide = SO₂
If they form magnesium oxide (MgO) and sulphur (S), these will be the product. The balanced chemical reaction is shown below:
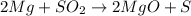
This gives the balanced equation for the reaction