157.64 grams of potassium permanganate contain 2.40x10^24 oxygen atoms.
1st) We need to know how many moles of oxygen are equivalent to 2.40x10^24 oxygen atoms. Here, we need to use the Avogadro's number (6.022x10^23) and with a mathematical Rule of Three we can calculate the moles of oxygen:
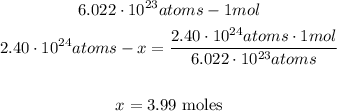
Now we now that there are 3.99 moles of oxygen.
2nd) The formula of potassium permanganate is KMnO4, so there are 4 moles of oxygen in the molecule, and the molar mass of potassium permanganate is 158.034 g/mol.
With a mathematical Rule of Three we can calculate how much potassium permanganate it is necessary for 3.99 moles of oxygen:
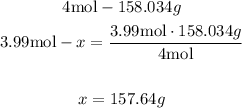
If 4 moles of oxygen are contained in 158.034g of potassium permanganate, then the 3.99 moles of oxygen will be contained in 157.64g of potassium permanganate.