Step 1 - Understanding the relation between temperature and pressure
There are three main variables that can modify the state of a gas: pressure (P), volume (V) and temperature (T). When one of them is kept constant, linear relationships arise between the remaining two.
So when we keep the volume constant (isovolumetric transformation), the pressure and the temperature become directly proportional, i.e., the greater the temperature, the greater the pressure and vice-versa.
We can state it mathematically as:
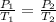
Step 2 - Using the equation to solve the question