Answer:
0.753 grams of copper metal are required to react with 4.00 grams of silver nitrate to produce copper (II) nitrate
Step-by-step explanation:
mass = moles * Mr
Equation of this reaction:
Cu(s) + 2AgNO₃(aq) → Cu(NO₃)₂(aq) + 2Ag(s)
---------------------------------------------------------------------------------------------
Find moles of silver nitrate:
4 = moles * 170
→
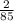 moles
moles
- Here we "2" molar ratio before silver nitrate and "1" before copper metal, so copper metal has half the moles of silver nitrate. copper metal has
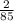 ÷ 2 =
÷ 2 =
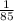 moles
moles
so find the mass of copper metal:
mass =
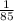 * 64
* 64
mass of copper is 0.753 grams.