Approximately 0.2851 liters or 285.1 ml of 5 M KOH needs to be added to achieve a pH of exactly 10.0 in the solution containing 1.0 L of 0.1 M glycine at pH 9.0.
To solve this problem, we'll use the Henderson-Hasselbalch equation, which relates the pH of a buffer solution to the pKa of the weak acid and the ratio of its conjugate base to weak acid. The equation is:
![\[ \text{pH} = \text{pKa} + \log\left(\frac{[\text{A}^-]}{[\text{HA}]}\right) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/s01p2secc4zqczpeehqr9bkjq0hqnj49tx.png)
Given that glycine is a diprotic amino acid and has two dissociation constants (pKa values: 2.35 and 9.78 for its carboxyl and amino groups, respectively), we'll consider the second pKa value (9.78) since the pH is closer to this value.
At pH 9.0, the solution contains both the glycine and its conjugate base in a specific ratio. We want to increase the pH to 10.0 by adding a strong base (5 M KOH), which will react with glycine's acidic groups and convert them to their conjugate bases.
To achieve this change in pH, we'll calculate the change in concentration of the conjugate base needed, then use the amount of 5 M KOH required to achieve this change.
First, let's determine the ratio of the conjugate base
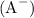 to the weak acid
to the weak acid
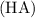 required to change the pH from 9.0 to 10.0 using the Henderson-Hasselbalch equation.
required to change the pH from 9.0 to 10.0 using the Henderson-Hasselbalch equation.
![\[\text{pH} = \text{pKa} + \log\left(\frac{[\text{A}^-]}{[\text{HA}]}\right)\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/a1l0mcdoz2t2wgwjufh8wkbzoegkegjr3p.png)
![\[10.0 = 9.78 + \log\left(\frac{[\text{A}^-]}{[\text{HA}]}\right)\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/3n6nki2zfyockpo7y8odyng4alf45mmpp9.png)
![\[\log\left(\frac{[\text{A}^-]}{[\text{HA}]}\right) = 10.0 - 9.78 = 0.22\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/op5xx6dkx260uyeut7pvt7vpxbry1virye.png)
![\[\frac{[\text{A}^-]}{[\text{HA}]} = 10^(0.22)\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/k3e98d6aej29caxgz2pv2j36w7xx202bq5.png)
![\[\frac{[\text{A}^-]}{[\text{HA}]} ≈ 1.584\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/5gkom3uffiuzydek8rmktvm4mi030pib2a.png)
Now, let's determine the initial concentration of glycine
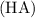 in the 1.0 L solution at pH 9.0 using its molarity (0.1 M) and the dissociation equation for glycine:
in the 1.0 L solution at pH 9.0 using its molarity (0.1 M) and the dissociation equation for glycine:
![\[\text{pH} = \text{pKa} + \log\left(\frac{[\text{A}^-]}{[\text{HA}]}\right)\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/a1l0mcdoz2t2wgwjufh8wkbzoegkegjr3p.png)
![\[9.0 = 9.78 + \log\left(\frac{[\text{A}^-]}{0.1}\right)\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/w4kpei42j4qb5cwvurjvu6gccm1t10ef7d.png)
![\[\log\left(\frac{[\text{A}^-]}{0.1}\right) = 9.0 - 9.78 = -0.78\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/fw7lysntiogkeeypejtdkidfacz4ufob9k.png)
![\[\frac{[\text{A}^-]}{0.1} = 10^(-0.78)\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/bxfi1ca1jjs5ogy69dkuwk21y9uf5wmfjk.png)
![\([\text{A}^-] ≈ 0.1584 \, \text{M}\)](https://img.qammunity.org/2024/formulas/chemistry/high-school/g9e7i0w026d1xqr2ud4akp8afcylw83saj.png)
Now, the change needed is the difference in the concentrations of
 at pH 10.0 and 9.0:
at pH 10.0 and 9.0:
![\(\Delta[\text{A}^-] = [\text{A}^-]_{\text{final}} - [\text{A}^-]_{\text{initial}}\)](https://img.qammunity.org/2024/formulas/chemistry/high-school/u8awy8c1v3a75ibnhnam48wsxixezpe3dp.png)
![\(\Delta[\text{A}^-] = 1.584 \, \text{M} - 0.1584 \, \text{M}\)](https://img.qammunity.org/2024/formulas/chemistry/high-school/6no4tz77ythgpzvle1ioeu5tvn12fq7qyw.png)
![\(\Delta[\text{A}^-] ≈ 1.4256 \, \text{M}\)](https://img.qammunity.org/2024/formulas/chemistry/high-school/a3jrj15bp0i8zzcl95y3zkd04wql88xgvw.png)
Now, to find the volume of 5 M KOH needed to provide this amount of
 , we'll use the equation:
, we'll use the equation:
![\[M_1V_1 = M_2V_2\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/hd7535hpbtoxjesqwp74g9kduyhqgz381f.png)
Given that the concentration of KOH is 5 M:
![\[5 * V_{\text{KOH}} = 1.4256\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/en6oms531y4w2uy1rdgarkoxexrj7zi9oj.png)
![\[V_{\text{KOH}} \approx 0.2851 \, \text{L}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/yy9bxexmxmbgskcz43oj4r8hojqhwqt3vu.png)