Answer:
Step-by-step explanation:
Answer: B
First we need a plan to solve this problem. Ultimately we want to get to our equation of molarity= moles of solute ÷ volume of solution (L). In order to get the volume of solution, we can use: Density = mass ÷ volume. So, 0.974g/mL=
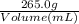 . Then we can solve for volume and get 272mL of solution. We then need to convert mL to L in order to use this in our molarity equation so 272mL(
. Then we can solve for volume and get 272mL of solution. We then need to convert mL to L in order to use this in our molarity equation so 272mL(
 ) = 0.272L of solution.
) = 0.272L of solution.
Now we need to find moles of NH3 by converting grams to moles using molar mass. The molar mass of NH3 is 17.031g/mol and we have 15.0 grams. Therefore 15.0g(
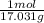 ) = 0.881mols of NH3.
) = 0.881mols of NH3.
Finally we can plug this into our molarity equation: Molarity =
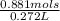 = 3.14mols/L of NH3.
= 3.14mols/L of NH3.