Approximately
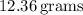 of
of
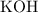 is left over as the excess reactant after the reaction.
is left over as the excess reactant after the reaction.
To determine the excess reactant and the limiting reactant, we need to calculate the number of moles of each reactant using their molar masses and the balanced chemical equation.
The balanced chemical equation for the reaction between potassium hydroxide
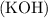 and iron (III) nitrate
and iron (III) nitrate
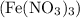 is:
is:
![\[\text{KOH} + \text{Fe}(\text{NO}_3)_3 \rightarrow \text{KNO}_3 + \text{Fe}(\text{OH})_3\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/rb6mtd4kpg4n2rcr8wm26wqhyhje2madbc.png)
The molar masses are:
-
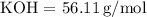
-
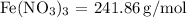
First, determine the number of moles for each reactant:
For
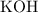 :
:
![\[\text{Moles of KOH} = \frac{\text{mass}}{\text{molar mass}} = \frac{17.0 \, \text{g}}{56.11 \, \text{g/mol}}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/knvg5ew3idhowh37ky5a0wwso2fn5emrgl.png)
For
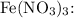
![\[\text{Moles of Fe}(\text{NO}_3)_3 = \frac{\text{mass}}{\text{molar mass}} = \frac{20.0 \, \text{g}}{241.86 \, \text{g/mol}}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/f6dinrcxcgvojqntldiucbc76y2hnmywuz.png)
Then, compare the mole ratios based on the balanced equation to identify the limiting reactant.
The balanced equation shows a 1:1 mole ratio between
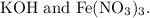
- Moles of KOH:
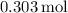
- Moles of Fe
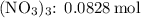
Since the moles of
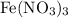 are less than the moles of
are less than the moles of
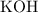 and both have a 1:1 ratio,
and both have a 1:1 ratio,
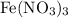 is the limiting reactant.
is the limiting reactant.
To determine the excess amount of left over, us
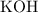 e the limiting reactant amount to find the actual amount of KOH used in the reaction.
e the limiting reactant amount to find the actual amount of KOH used in the reaction.
Moles of
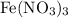 used in the reaction
used in the reaction
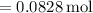
Since the mole ratio is 1:1, the moles of
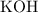 used are also
used are also
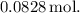
Therefore, the excess
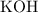 left over is:
left over is:
![\[\text{Excess } \text{KOH} = \text{Total moles of KOH} - \text{Moles of KOH used}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/575wdxj10wf8phxk953p4gezhdsqtap1vw.png)
![\[\text{Excess } \text{KOH} = 0.303 \, \text{mol} - 0.0828 \, \text{mol}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/fc88c6arz9u3szxxks91uhyzfl5xx6zwc7.png)
![\[\text{Excess } \text{KOH} = 0.2202 \, \text{mol}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/duk2qdk7bvy0q3ajv7ds5uxgxuxodefaj5.png)
Finally, calculate the excess mass of
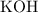 left over using the molar mass:
left over using the molar mass:
![\[\text{Excess mass of KOH} = \text{Excess moles} * \text{Molar mass of KOH}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/1cep5fn2621tjkp2qc3ahqe3owm5mp9rqt.png)
![\[\text{Excess mass of KOH} = 0.2202 \, \text{mol} * 56.11 \, \text{g/mol}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/qn2376lnpu1q15o0vp0jldjezyueegl6da.png)
![\[\text{Excess mass of KOH} \approx 12.36 \, \text{g}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/jkxdr88oueccbzs1j73xke4arjghyn4ro1.png)
Question:
How much excess reactant is left over when
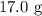 of potassium hydroxide
of potassium hydroxide
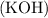 reacts with
reacts with
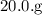 of iron (III) nitrate
of iron (III) nitrate
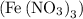 ?
?