Answer:
0.001525 mol
Step-by-step explanation:
To find the number of moles of phosphorous in 0.25 g of Na₃PO₄, we will first calculate the molar mass of Na₃PO₄, then use stoichiometric ratios based on its molecular formula, and finally apply the given mass to determine the moles of phosphorous.

Step (1): Determine the molar mass of Na₃PO₄
1 mole of Na₃PO₄ consists of:
- 3 moles of Na (Sodium)
- 1 mole of P (Phosphorus)
- 4 moles of O (Oxygen)
Atomic weights:
- Na (Sodium) = 22.990 g/mol
- P (Phosphorus) = 30.974 g/mol
- O (Oxygen) = 15.999 g/mol
Using atomic weights to determine the molar mass of Na₃PO₄:
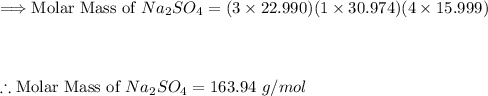
Step (2): Using stoichiometry
From the molecular formula, Na₃PO₄, it is evident that:
1 mole of Na₃PO₄ contains 1 mole of Phosphorus (P)
Step (3): Calculate the moles of phosphorus from 0.25 g of Na₃PO₄
Using the formula:
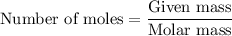
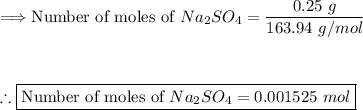
There are 0.001525 moles of phosphorous in 0.25 g of Na₃PO₄.